


|

|
|---|
| Communication | Newsletter | About Mordants | Symposia | Instructors | Contact us! |
|---|
Many natural dyes are enhanced by and made more permanent with the use of "mordants". Mordants are "metallic salts" that are used in natural dyeing to help set the dye pigment and improve color and light fastness. The word comes from the Latin word "mordere" meaning "to bite".
The fibers are usually pre-mordanted ahead of time, by simmering the yarn in a carefully measured bath of water and metallic salt for about an hour. When the fiber is immersed in the hot mordant bath, the metallic salts bond directly to the strands of fiber. When the mordanted fiber is later put into a dye bath, the pigment molecules form a chemical bond with the mordant on the fiber, thereby setting the color and increasing its ability to be lightfast and colorfast.
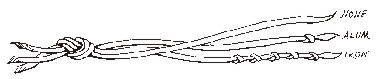
In her first mushroom dye book, "Let's Try Mushrooms for Color", (Thresh Publications, 1974) Miriam C. Rice introduced the concept of a knotting scheme in order to differentiate which samples of yarn had been pre-mordanted with which mordants. This is an important tool if yarn skeins mordanted with several different mordants are immersed in the same dye bath. This knotting scheme is almost universally used today by dyers when using mushroom and other natural dyes.
Originally, Miriam used 5 different mordants, each assigned different knots:
No mordant = 0 (no knot)
Potassium aluminum sulphate (ALUM) = 1 knot
Potassium dichromate (chrome) = 2 knots (see articles below)
Stannous chloride (tin) = 3 knots
Copper sulphate (copper) = 4 knots
Ferrous sulphate (IRON) = 5 knots
Over the years, and after much research, Miriam has decided that the only SAFE mordants to use are ALUM and IRON. But the knotting system has remained the same - 1 for alum and 5 for iron - a reminder of why the other knots are missing!
Miriam recommends checking out the "MSDS" information (Materials Safety Data Sheets) of these materials, if you have any question about their cautions and uses.
About Mordants - Then and Now...
For many years now, due to growing awareness of the toxic nature of some of the mordants we have used in the past (tin, chrome, and copper), our experimentation has shifted to the use of only ALUM and IRON mordants for mushroom dyes. This choice is for our own safety as well as a contribution towards a less polluted biosphere. Results recorded for more than a decade now show tlittle need for dependence on toxic mordants in order to achieve the broad diversity of a full color spectrum including the bright reds, yellows, blues and purples.
Please join us in this effort! " - Miriam C. Rice
 |
|||
|---|---|---|---|
| MUSHROOM SPECIES | No Mordant (" no knot " ) |
Alum Mordant (" 1 knot " ) |
Iron Mordant (" 5 knots " ) |
 Phaeolus schweinitzii
Phaeolus schweinitzii |
|||
 Dermocybe phoeniceus
var. occidentalis
Dermocybe phoeniceus
var. occidentalis |
|||
 Omphalotus olivescens
Omphalotus olivescens |
|||
CHROME AND CHROMATES
by Dr. Erik Sundström, Sweden
Sodium or potassium dichromates have been suggested as mordants, ever since they were used with the first synthetic mauvein dye in 1856. Potassium dichromate was preferred, since sodium dichromate absorbs moisture and becomes slimy. The dichromates are orange to brownish red and will stain the wool themselves without helping the natural dyes to fasten, and they may produce interesting color combinations.
Water soluble chrome compounds are generally either green chrome(III) salts where the chrome is the positive ion, or chromates where the negative ion is chrome(VI) with oxygen. Chromates were once regarded as innocent and even tried as medicines, but in the 1970's they were found to be so dangerous and the occupational health regulations became so severe, that they may only be handled in closed systems by personnel in full protective suits.
The main reason is that the cells of our body are only prepared to handle water soluble ions of the kinds occurring in nature. This means among negative ions: carbonate, sulphate, chloride and hydroxyl, but not chromate, and among positive ions hydrogen, ammonia and most metals except lithium, caesium and some of the heaviest like lead, cadmium or mercury. The cell walls will keep an equilibrium by shuttling sodium, potassium, magnesium and calcium back and forth, and failure to do so leads to nervous problems. Among other metals, iron is needed for blood and muscles in rather large amounts, and zinc in small amounts for tissue repair. Metals which could be mistaken for iron must be kept out so as not to disturb the iron process, and among them are copper and chrome, and tin will be kept out so it will not be mistaken for zinc.
There is no defense against chromate ions, however, since they do not occur in nature, and they can easily enter into the cells. Once they get in, they can attach as mordants to protein to make it water repellent. Chromates are also strongly oxidizing with their oxygen burning or breaking other cell component.. When this occurs, chrome(VI) turns to chrome(III) which disturbs the action of iron. By then the immune system becomes aware that something is wrong, but has no indication of any dangerous stuff passing through the cell membrane, so it starts looking for some probable enemy to fight. You may then become allergic to something close-by, but not chromates. If you are a dyer you might become allergic to wool, a tanner might be allergic to leather, etc. Chromates are also carcinogenic and may start fires. They will also disturb the micro-organisms in water purification plants, so it is not permitted to dump chromates in the drains.
(Dr. Erik Sundström, has a Doctorate in the Science of Materials and is a Mycologist. He is co-author of
"Färga med svampar", and the author of a new Swedish book about pigments of Natural Dyes.)
WHY WE DON'T USE CHROME ANYMORE!
by Darvin DeShazer, USA
###########################
Caution Advised!
Chrome hazards are lurking in the mordant potassium dichromate.
Have you ever poured your old, used motor oil into a gopher hole? Do you bury plastic or styrofoam in your backyard? Do you dump toxic metals into the environment? Do you even CARE about our Mother Earth? You might have answered YES to a couple of these questions and that would be one too many!
Simply stated: Some of our natural dye friends are dumping toxic metals into the environment.
For millennia, dyers and weavers have searched and experimented with plants, minerals and other colored materials for ways to impart the color to their work. The bright red-orange color of potassium dichromate has proven to work well. The name chromium comes from the Greek word for color, chroma.
The characteristic oxidation states of the metal (+2, +3 or +6) yield several different colors: blue to violet to green to orange. The change in oxidation state leads to confusion over the proper name but dyers are interested in only one of the compounds, potassium dichromate because of its color stability. It¹s a salt of Chromic Acid and has the chemical formula of K2Cr2O7. It's also known as Hexavalent chromium ("chromium VI"), Cr(VI), Cr+6, Dichromic Acid, Dipotassium Salt, Potassium Bichromate and Dipotassium
Dichromate.
Medically, it is well known to irritate the skin and mucous membranes. Direct contact may cause skin irritation, sensitization or dermatitis. Prolonged contact can cause external ulcers, known as "Chrome Sores". Chrome sores most commonly occur at breaks in the skin, nailroots, creases over knuckles, finger webs, backs of hands, and on forearms. Massive overexposure could lead to toxic quantities being absorbed through the skin causing systemic poisoning which leads to kidney or liver damage. Initial poisoning by potassium dichromate may cause vomiting, pain in the stomach, and a metallic taste. Circulatory collapse may follow with weak and rapid pulse, shallow respiration, and clammy skin. Early deaths are generally associated with shock. Late deaths are usually due to renal or hepatic failure. Chrome(VI) can kill humans and is known to cause cancer.
Mild inhalation of the dust may irritate the respiratory tract and can be a major problem for natural dyers. Symptoms include coughing, shortness of breath, sore throat and a runny nose. If sufficient amounts are inhaled and absorbed, symptoms may resemble those caused by acute ingestion. Ingestion may cause gastroenteritis (inflammation of the lining membrane of the stomach and intestines) with abdominal pain, nausea, vomiting and diarrhea.
This may result in systemic effects, which can occur anywhere in the body, and may include ringing of the ears, dizziness, elevated blood pressure, blurred vision, tremors and problems with the kidneys and liver. Dyers would most likely never be poisoned by inhaling the small misty droplets from a boiling dye pot and ingestion is out of the question. They are at risk when removing a tablespoon full of the powder from the container and adding it to the dye pot. Inhalation of dichromate dusts can cause ulceration and perforation of the nasal septum, irritation of the eyes and respiratory tract problems. This can be minimized with education, such as holding your breath while using an open container of the crystals and boiling the dye pot in a well ventilated area.
The other REAL problems with using potassium dichromate are skin contact and disposal of the left over water. Contact with breaks in the skin cause ulcerations, known as chrome sores. They were common during the industrial revolution, before the focus on safety in the work place. Wool that has been dyed with a mordant containing chrome will have some chrome in the fibers of the wool, so it is prudent to avoid wearing the clothing. That would be analogous to wearing a chrome plated bumper from a '57 Chevy! Shiney chrome plated jewelry for children (or anyone) is against the law! Wool dyed with chrome as a mordant would be suitable for wall hangings and pieces of art, but not clothing, especially for children and pregnant women. NEVER make a child's hat or sweater with chromated wool!
Dr. Gale was able to produce cleft palates in 85% of the hamster fetuses he tested with a dose of 7.5 mg/kg of chromium. That is about 75 times the exposure limit allowed by US Federal Safety Authorities. For Cr+6, the lethal dose is 50 mg/kg of body weight for humans. For a typical adult female weighing 132 pounds (60 kg), the fatal dose would be 3.00 grams. Most jars contain 500 grams! A 60 kg crafts dyer would only need 0.0064 g to exceed the federal guidelines.
AVOID DIRECT CONTACT WITH THIS MATERIAL. Do not eat, drink or smoke in areas where potassium dichromate is being used.
The last problem involves disposal of the spent dye bath water. The water contains chromium VI and will reside in the soil for over a decade before binding or filtering out. Eventually it changes chemically into chromic oxide, Cr2O3, and becomes a safer mineral with minimal health risks.
A Google search for "hazards of potassium dichromate" returned over 1250 web sites. Could ALL OF THEM possibly be wrong? Play it safe, for your health, your children, your caring neighbors and the future generations. Avoid using potassium dichromate as a mordant, don't dump the water into the sewer and feel good about helping to save Mother Earth.
Darvin DeShazer majored in Biology and has teaching credentials in Life Science and Chemistry. He is cofounder of SOMA (Sonoma County Mycological Association), and has served as the first President, a former newsletter editor, Webmaster and Science Advisor. He assists hospitals and veterinarians with mushroom identification and is a consultant for the Bay Area Poison Control Center and the Univ. of California Agricultural Extension Service. Darvin has authored several publications about fungi, teaches workshops in fungal microscopy, collects autographed mushroom books and is the Chairman of the Science Department at St. Vincent High School, Petaluma, CA.
Both of the above articles first appeared in the IMDI Newsletter "The Dye-Gest"
|